A Controlled-Release Oral Pill for Kala Azar and Fungal Infections
- News
- 1.5K
Researchers at Indian Institute of Technology Hyderabad have developed a method to produce controlled-release oral pills for treating fungal infections and kala-azar. The tablets were found to release the drug Amphotericin B in a sustained and controlled manner over a period of 10 days.

Amphotericin B is the drug of choice for fungal infections of the abdomen and heart valves, fungal pneumonia, and kala-azar. However, it is costly, causes toxicity and its bioavailability is poor. The drug is administered through injection, which results in the uncontrolled release into the bloodstream.
The researchers have developed the oral tablet by loading the drug on to gelatin nano-fabric. They chose gelatin since it is non-toxic and biocompatible, besides being biodegradable. It is used in the food and medical industries.
The drug was loaded on to gelatin and was then drawn out in the form of nanofibers through the process of electrospinning. It involves the use of electric force to draw out charged threads of polymers into fibers with diameters in the order of some hundred nanometers. In order to improve structural consistency in aqueous conditions, saturated vapors of a chemical called glutaraldehyde were used.
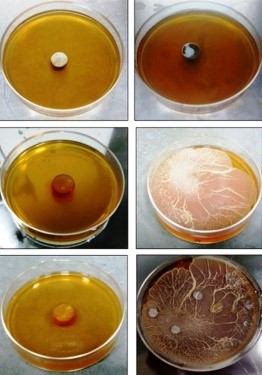
The team has also found that the nano-fibrous oral tablet was stable over a wide range of pH, which means that it would not be destroyed in the gastrointestinal tract before drug absorption into the bloodstream.
Explaining the rationale for using nanofibres to produce the oral tablets, researchers said, “nanofibers – fibers that are a hundred thousand times thinner than the human hair – are increasingly being considered as a medium for controlled release of drug molecules because of their large surface area and porosity”.
The research was led by Prof. Saptarshi Majumdar and Dr. Chandra Shekhar Sharma. The study results have been published in journal Nano-Structures and Nano-Objects. The paper has been co-authored by Dr. Anindita Laha, Ms. Mrunalini Gaydhane. (ISW)
If you liked this article, then please subscribe to our YouTube Channel for the latest Science & Tech news. You can also find us on Twitter & Facebook.