Cancer Cells Manipulate Immune System for Their Survival
- News
- 2.7K
Researchers have found that cancer cells in the body manipulate the immune system for their survival and expansion.
Cancer arises primarily because of immune system dysfunctions. Prevalence of an immune-suppressive environment increases the likelihood of tumor growth and progression. T lymphocytes especially, T helper cells (Th) and T cytotoxic cells (Tc) play crucial roles in shaping our immune response against foreign bodies as well as cancer cells.
However, there is another subset of T cells, which are immunosuppressive in nature, known as regulatory T cells. Their function is to protect the body from autoimmune disorders. In a healthy individual, there is a well-defined balance between helper T cells and regulatory T cells that determines the success of immune surveillance over immune suppression.
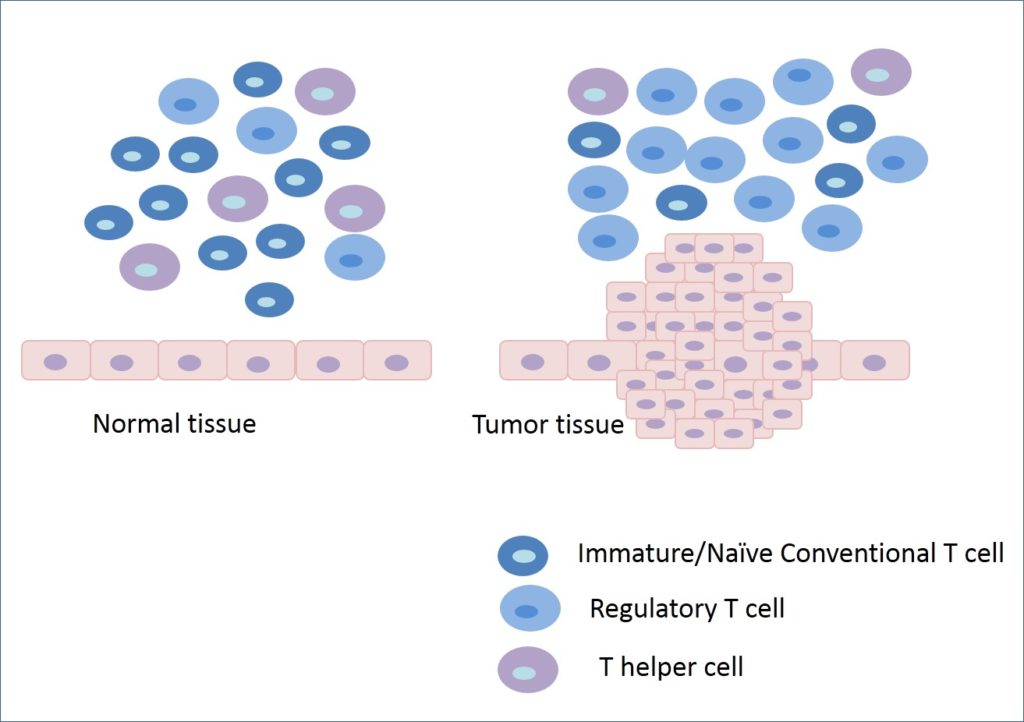
Cancer cells often find out several ways to evade the immune system. One of the strategies of cancer cells is to encourage the selective proliferation of immunosuppressive regulatory T cells in the tumor microenvironment. In fact, tumor cells send signals to immature conventional T cells for their selective differentiation and proliferation into regulatory T cells. In this way, an immunosuppressive environment is maintained, and eventually, the tumor escapes the attack from the host immune system.
Though several reports indicate that there is an abundance of regulatory T cells in the tumor microenvironment, the cues into their metabolism enabling selective proliferation were not much discussed. However, in a recent study, published in Proceedings of the National Academy of Sciences, scientists have shown that preferential proliferation of regulatory T cells in the tumor microenvironment is facilitated by metabolic reprogramming.
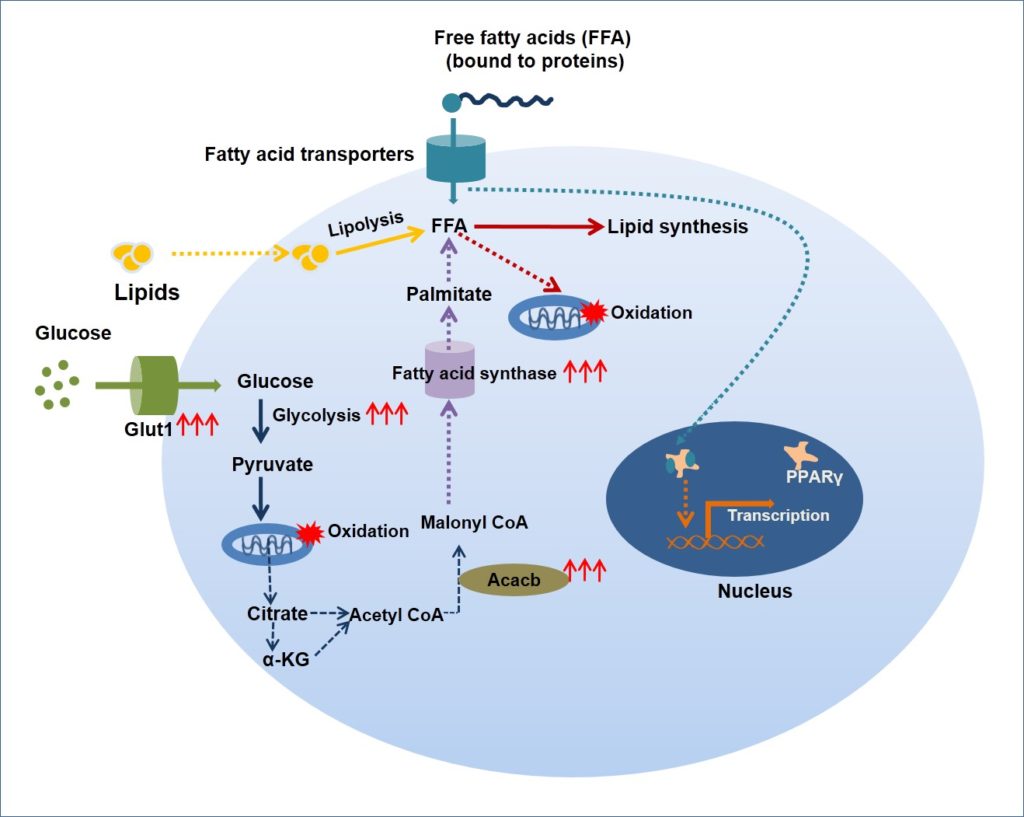
Metabolic reprogramming in cancer cells has been a much-discussed topic. Cancer cells adapt themselves to aerobic glycolysis and de novo lipogenesis, since both these pathways provide sufficient building blocks for proliferating cells, apart from providing energy. The current study shows that regulatory T cells undergo selective proliferation facilitated by a similar reprogramming of lipid and glucose metabolism.
The study shows that tumor-associated regulatory T cells can accumulate intracellular lipids. These cells over-express proteins such as Acetyl-CoA carboxylase β (Acacb) and Peroxisome proliferator-activated receptor gamma (PPARγ) for enhanced fatty acid synthesis and storage.
The Acacb enzyme catalyzes the carboxylation of acetyl-CoA to malonyl-CoA, the rate-limiting step in the fatty acid synthesis. PPARγ activated by long-chain saturated and unsaturated fatty acids regulate the expression of genes encoding proteins that regulate fatty acid storage and oxidation.
Glucose uptake in regulatory T cells is facilitated by over-expression of Glut1. The rate of glycolysis is enhanced by several folds in these cells, as indicated by the overexpression of several glycolysis-associated genes. Further, the metabolic intermediates of Krebs cycle such as citrate and α-ketoglutarate can also act as substrates for de novo fatty acid synthesis. The authors claim that both fatty acid metabolism and glycolysis enable selective regulatory T cell expansion during tumor growth.
Since the abundance of regulatory T cells in the tumor site bears a direct correlation to the complexity and heterogeneity of tumor, they may be an interesting target in cancer therapy. The current study shows that an in-depth understanding of immuno metabolomic profiles has great implications in determining the success of cancer therapy. Hopefully, these insights may enable a paradigm shift in the conventional cancer treatment strategies.
The study has been published in the journal Proceedings of the National Academy of Sciences as ‘Fatty acid metabolism complements glycolysis in the selective regulatory T cell expansion during tumor growth. Proc Natl Acad Sci U S A. 2018 Jul 10; 115(28):E6546-E6555′.
By Dr. Archana Payickattu
Image Credit – Wikimedia Commons
If you liked this article, then please subscribe to our YouTube Channel for the latest Science & Tech news. You can also find us on Twitter & Facebook.